Which statement about niels bohr atomic model is true
Home » Query » Which statement about niels bohr atomic model is trueYour Which statement about niels bohr atomic model is true images are available. Which statement about niels bohr atomic model is true are a topic that is being searched for and liked by netizens today. You can Download the Which statement about niels bohr atomic model is true files here. Get all free photos and vectors.
If you’re searching for which statement about niels bohr atomic model is true pictures information connected with to the which statement about niels bohr atomic model is true keyword, you have come to the ideal blog. Our website always provides you with hints for downloading the highest quality video and image content, please kindly search and find more enlightening video content and images that match your interests.
Which Statement About Niels Bohr Atomic Model Is True. A change in which property of light will have no effect on whether or not the photoelectric effect occurs. Which statement about niels bohrs atomic model is true. Click card to see definition. Although he arrived at his model and its principles in collaboration with the august founder of the atomic nucleus Ernest Rutherford the model is only credited to BohrOriginally called the Rutherford-Bohr atomic model it is now commonly referred to as Bohrs atomic model.
 Niels Bohr Physicist Who Took A Quantum Leap Quantum Physics Quantum Mechanics Quantum Physics Spirituality From pinterest.com
Niels Bohr Physicist Who Took A Quantum Leap Quantum Physics Quantum Mechanics Quantum Physics Spirituality From pinterest.com
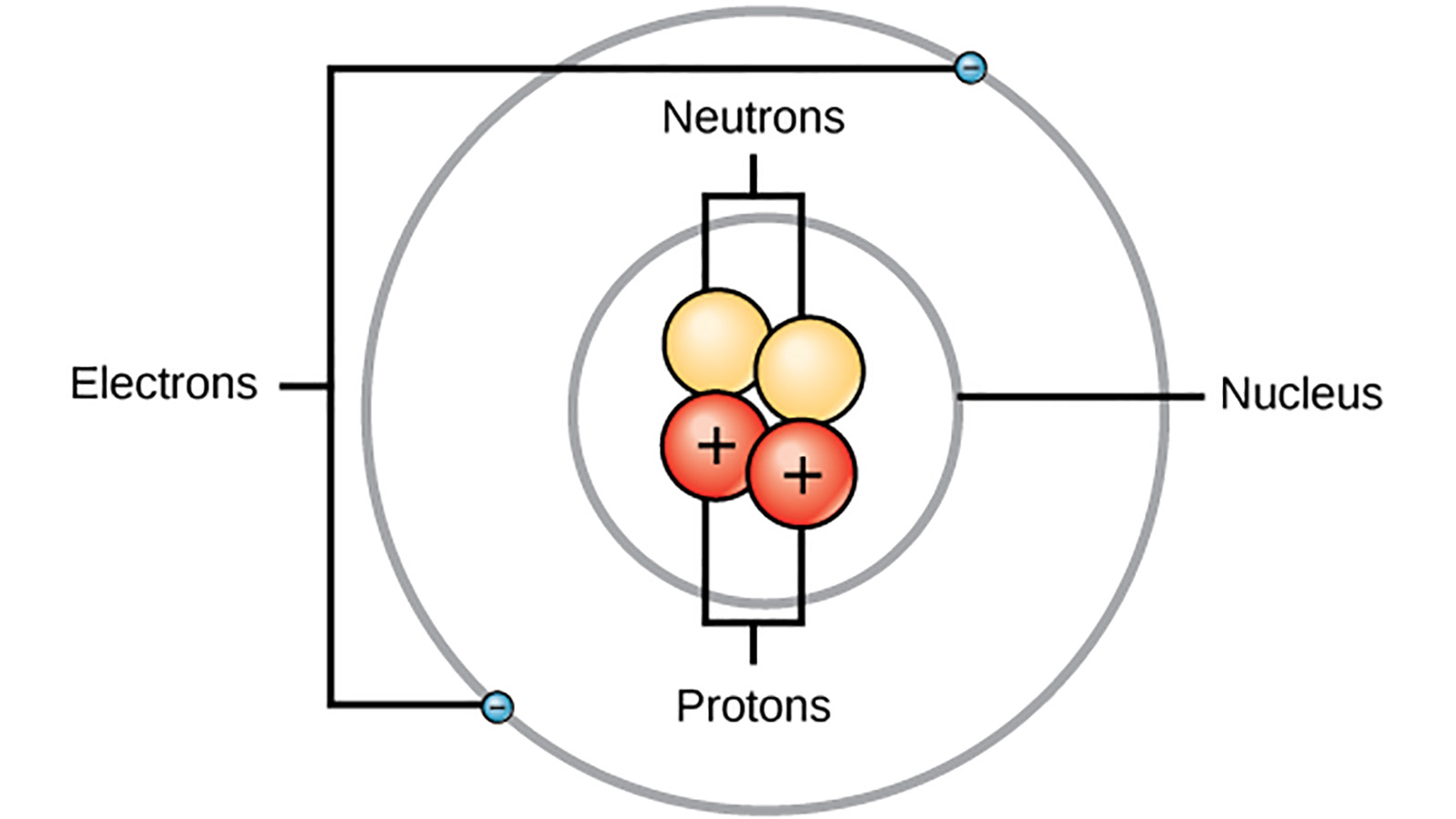
In the year 1913 Niels Bohr proposed an atomic structure model describing an atom as a small positively charged nucleus surrounded by electrons that travel in circular orbits around the positively charged nucleus like planets around the sun in our solar system with attraction provided by electrostatic forces popularly known as Bohrs atomic model. Another electron is elevated from level 2 to level 4. Click again to see term. Higher orbits have lower energies. Electrons can exist in any energy level. Niels Bohr was born in Copenhagen Denmark in 1885.
A change in which property of light will have no effect on whether or not the photoelectric effect occurs.
Higher orbits have lower energies. As the atomic number increases the melting and boiling. Which statement about Niels Bohrs atomic model is true. Bohr developed the Bohr model of the atom in which he proposed. Tap again to see term. Each orbit has a specific energy level.
 Source: pinterest.com
Source: pinterest.com
Truth and clarity are complementary. O Orbits close to the nucleus have no energy. Level 2 to level 4. O Higher orbits have lower energies. The transition requiring the greatest energy change is.
 Source: science.howstuffworks.com
Source: science.howstuffworks.com
Higher orbits have lower energies. A deep truth is a truth so deep that not only is it true but its exact opposite is also true. As the atomic number increases the melting and boiling. The transition requiring the greatest energy change is. Each orbit has a specific energy level.
 Source: expii.com
Source: expii.com
As the atomic number increases the melting and boiling. Quite simply Niels Bohr illuminated the mysterious inner-workings of the atom. Each orbit has a specific energy level. Higher orbits have lower energies. The model accounted for the absorption spectra of atoms but not for the emission spectra.
 Source: universetoday.com
Source: universetoday.com
He was also a keen philosopher and pioneer of scientific research especially in the field of physics. The model accounted for the absorption spectra of atoms but not for the emission spectra. Click card to see definition. Which statement about Niels Bohrs atomic model is true. Electrons can exist in any energy level.
 Source: 8sa.net
Source: 8sa.net
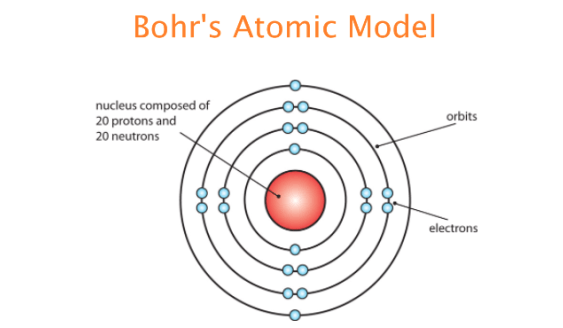
Though the Bohr atomic model also describes the relationship between the energy and size of the orbital which says that the smallest orbital has the lowest energy. Click again to see term. Tap card to see definition. A hydrogen electron is elevated from level 1 to level 2. He modified the problems and limitations associated with Rutherfords model of an atom.
 Source: pinterest.com
Source: pinterest.com
Which statement about Niels Bohrs atomic model is true. O Higher orbits have lower energies. Light is emitted by electrons when they drop from one energy level to a lower level. A change in which property of light will have no effect on whether or not the photoelectric effect occurs. It is the hallmark of any deep truth that its negation is also a deep truth.
 Source: britannica.com
Source: britannica.com
He modified the problems and limitations associated with Rutherfords model of an atom. Click again to see term. Autumnlatuch autumnlatuch 04062020 Chemistry Middle School answered Which statement about Niels Bohrs atomic model is true 2 See answers Advertisement Advertisement litman11 litman11 AnswerB. The model accounted for the absorption spectra of atoms but not for the emission spectra. They exist at specific energy levels.
 Source: pinterest.com
Source: pinterest.com
Each orbit has a specific energy level. A change in which property of light will have no effect on whether or not the photoelectric effect occurs. Which statement about Niels Bohrs atomic model is true. Which statement about Niels Bohrs atomic model is true Get the answers you need now. Another electron is elevated from level 2 to level 4.
 Source: chemed.chem.purdue.edu
Source: chemed.chem.purdue.edu
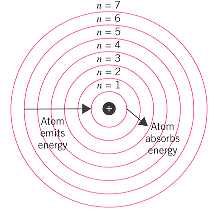
Each orbit has a specific energy level. Another electron is elevated from level 2 to level 4. Orbits close to the nucleus have no energy. Correct answer to the question Which statement about Niels Bohrs atomic model is true. They exist at specific energy levels.
 Source: pinterest.com
Source: pinterest.com
As the atomic number increases the melting and boiling. Which statement about niels bohrs atomic model is true. A change in which property of light will have no effect on whether or not the photoelectric effect occurs. What is true of valence electrons. Click card to see definition.
 Source: pinterest.com
Source: pinterest.com
Each orbit has a specific energy level. In the year 1913 Niels Bohr proposed an atomic structure model describing an atom as a small positively charged nucleus surrounded by electrons that travel in circular orbits around the positively charged nucleus like planets around the sun in our solar system with attraction provided by electrostatic forces popularly known as Bohrs atomic model. Quite simply Niels Bohr illuminated the mysterious inner-workings of the atom. Bohr was able to predict the difference in energy between each energy level allowing us to predict the energies of each line in the emission spectrum of hydrogen and understand why electron energies are quantized. Tap again to see term.
 Source: pinterest.com
Source: pinterest.com
Niels Henrik David Bohr Danish. A hydrogen electron is elevated from level 1 to level 2. 7 October 1885 18 November 1962 was a Danish physicist who made foundational contributions to understanding atomic structure and quantum theory for which he received the Nobel Prize in Physics in 1922. Rutherford did not explain the relation of the orbital. He was a renowned scientist who won a Noble Peace Prize in 1922 for his work in understanding atomic structure and quantum theory.
 Source: pinterest.com
Source: pinterest.com
O higher orbits have lower energies. Truth and clarity are complementary. Another electron is elevated from level 2 to level 4. Niels Henrik David Bohr Danish. Which statement about Niels Bohrs atomic model is true.
 Source: expii.com
Source: expii.com
Which statement about Niels Bohrs atomic model is true. Level 2 to level 4. Bohr developed the Bohr model of the atom in which he proposed. A Danish physicist named Neil Bohr in 1913 proposed the Bohr atomic model. Another electron is elevated from level 2 to level 4.
 Source: id.pinterest.com
Source: id.pinterest.com
Which statement is true concerning Bohrs model of the atom. Each orbit has a specific energy level. There are trivial truths and. Tap card to see definition. He modified the problems and limitations associated with Rutherfords model of an atom.
 Source: pinterest.com
Source: pinterest.com
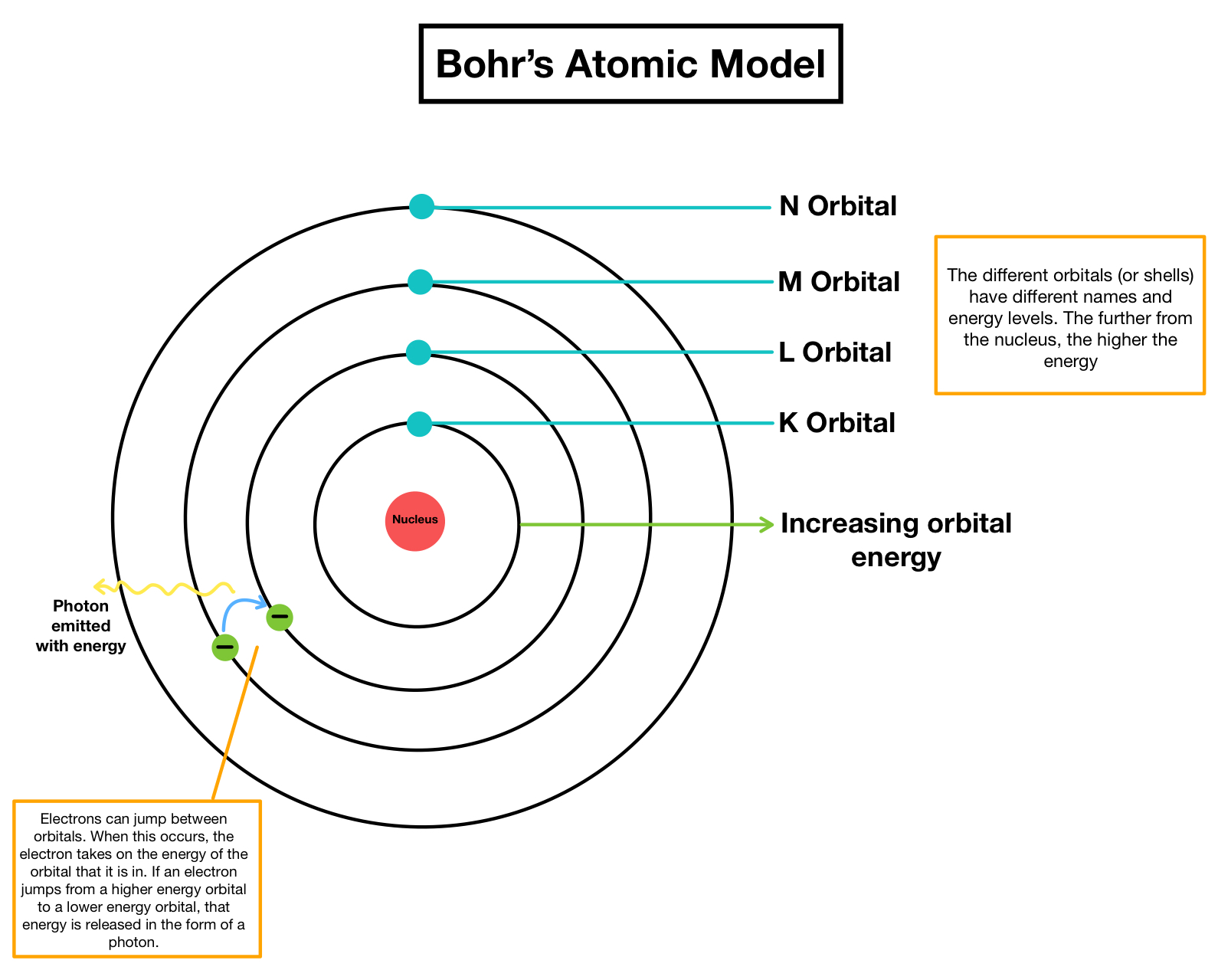
Light is emitted by electrons when they drop from one energy level to a lower level. O Each orbit has a specific energy level. What is true of valence electrons. Higher orbits have lower energies. Another electron is elevated from level 2 to level 4.
 Source: pinterest.com
Source: pinterest.com
O Each orbit has a specific energy level. Click card to see definition. The main problem with Bohrs model is that it works very well for atoms with only one electron like H or He but not at all for multi-electron atoms. Bohr was also a philosopher and a promoter of scientific research. Electrons can exist in any energy level.
 Source: expii.com
Source: expii.com
Bohr developed the Bohr model of the atom in which he proposed. Each orbit has a specific energy level. Shining bright blue light on a strip of metal. The model accounted for the absorption spectra of atoms but not for the emission spectra. There are trivial truths and.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title which statement about niels bohr atomic model is true by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.